Single molecule biophysics involves the investigations of individual biomolecules, such as proteins, DNA, RNA, and molecular motors, along with their interactions. Examining these molecules individually gives detailed insight into processes like protein folding, motor mechanics, enzyme kinetics, and intramolecular forces – information that is often challenging or even impossible to obtain from bulk studies.
Aresis optical tweezers, equipped with fluorescence microscopy and TIRF, are excellent for observing and studying biomolecular mechanics and interactions in real time. The system enables simultaneous generation of multiple traps, or a combination of optical traps with micropipettes, allowing for non-invasive manipulation with sub-nanometre precision.
In typical experiments, molecules under investigation are chemically bound to functionalised microspheres. Forces acting on the sphere are precisely controlled and monitored through their displacements in calibrated traps. The Aresis system features fully automated force calibration, ensuring reliable and reproducible force measurements on up to 20 particles simultaneously, with a resolution below 0.1 pN. An optional hardware module with a quadrant photodiode (QPD) is available for force measurements upon request.
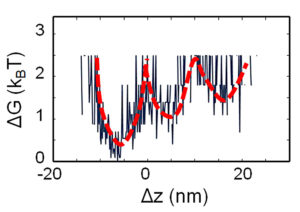
Example 1: Free energy landscape of single binding events
A combination of Aresis optical tweezers and plasmonic imaging was used to study molecular interactions and subsequently to determine the thermodynamic profile of the free energy landscape for the IgG/anti-IgG binding pair. The highly focused tweezers laser beam was employed to locally heat 150 nm gold nanoparticles to which anti-IgG molecules were attached. By measuring the free energy profiles of binding events at different temperatures, a detailed free energy landscape was obtained.
Selected related references:
Single-molecule calorimeter and free energy landscape; Yi Wang et al 2021 Proc. Natl. Acad. Sci. USA 118 e2104598118; DOI: 10.1073/pnas.210459811 LINK