Research in the field of cell mechanics explores mechanical properties of living cells and their relation to cell function. Most commonly, the mechanics of the cell is investigated either by applying external force or by sensing forces exerted by the cell. Aresis optical tweezers offer ultra-precise control for manipulation of living cells under physiological conditions while noninvasively measuring forces through video-based position detection.
Selection of one or multiple simultaneous traps with adjustable stiffness opens up the possibility of diverse applications, such as studying the response of cell membrane to complex external stimuli; tether pulling experiments; investigating the mechanisms behind cell motility; exploring interactions of cells with the environment in the form of adhesion; or identifying diseased cells via changes in their mechanical properties. The system’s compatibility with Nikon microscopes supports the use of standard microscope heating stages, keeping the cells in a controlled and stabilised environment, as well as selective fluorescent imaging.
Aresis optical tweezers are widely employed in cell mechanics experiments, as following examples show.
Example 1: Cell stiffness measurements by controlled microbead indentations
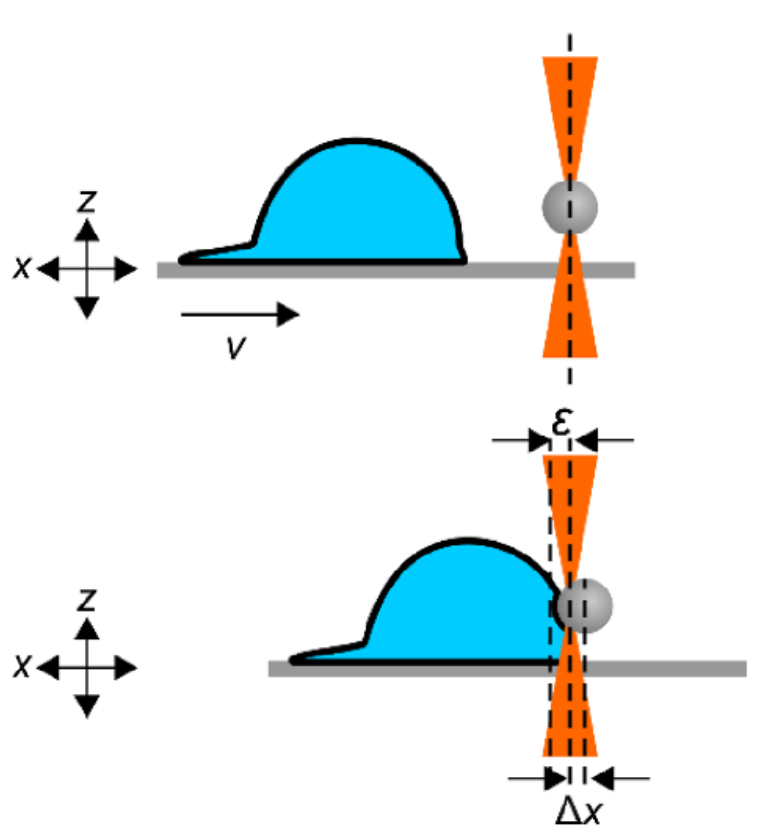
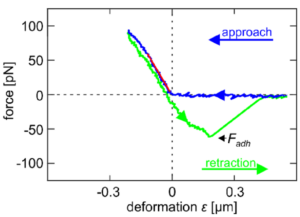
The effect of actin-disrupting drugs on cellular stiffness of human umbilical vein endothelial cells was studied by Aresis optical tweezers. Loading forces of 70-100 pN were exerted on the cell membrane and underlying cortex by trapping a 5 µm silica microsphere and laterally indenting the cells for about 200 nm. Precise video position detection system yielded force-deformation curves, from which cell stiffness was determined as the slope of the curve. Upon retraction, adhesion of the cell is detected by the tweezers and depicted as negative forces. The small indentation measurements show a significant decrease in cell stiffness after treatment with actin-disrupting drugs.
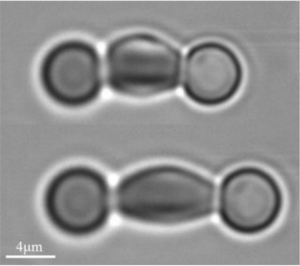
Aresis tweezers were use to probe erythrocyte membrane shear modulus: decline of elasticity was observed with passing in vitro time as well as an increase in shear modulus in diseased cells. In both cases, two microbeads were attached diametrically to the cell and the red blood cell was carefully stretched by applying force with optical tweezers. The pulling forces ranged from 2 pN to 8 pN, which resulted in an extension ratio of up to 40 %.
Selected related references:
Cell stiffness under small and large deformations measured by optical tweezers and atomic force microscopy: effects of actin disruptors CK-869 and jasplakinolide; Špela Zemljič Jokhadar et al 2021 J. Phys. D: Appl. Phys. 54 124001; DOI: 10.1088/1361-6463/abd0ae LINK
Cortical stiffness of keratinocytes measured by lateral indentation with optical tweezers; Špela Zemljič Jokhadar et al 2020 PLoS ONE 15(12): e0231606; DOI: 10.1371/journal.pone.0231606 LINK
In vitro investigation of the mechanics of fixed red blood cells based on optical trap micromanipulation and image analysis; Hongtao Rao et al 2024 Biomed. Opt. Express 15, 3783-3794; DOI: 10.1364/BOE.523702 LINK
Study of in vitro RBCs membrane elasticity with AOD scanning optical tweezers; Huadong Song et al 2017 Biomed. Opt. Express 8, 384-394; DOI: 10.1364/BOE.8.000384 LINK
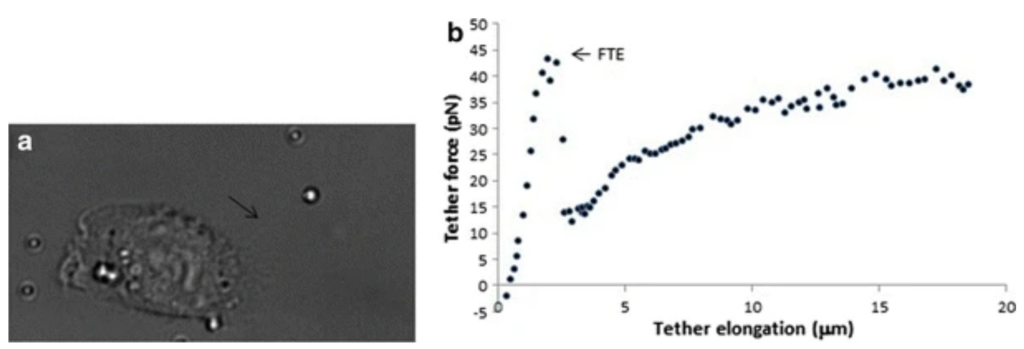
Example 2: Membrane tether extraction
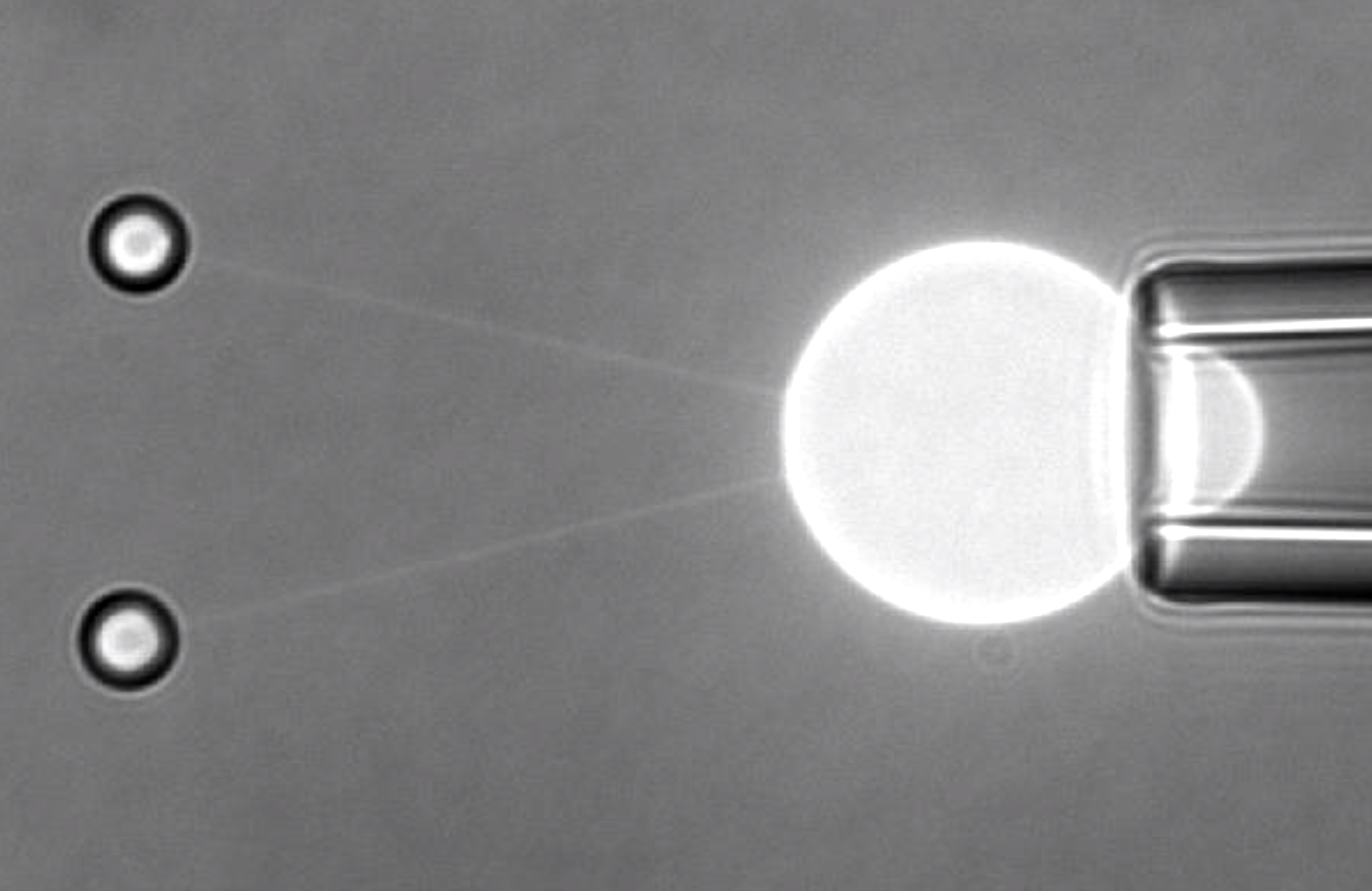
Tether extraction is possible by attaching individual microbeads to the membrane and pulling them with optical tweezers. Aresis tweezers precise control over position and movement of multiple optical traps enables extraction of several tethers simultaneously. For example streptavidine-coated microbeads were pulled from biotinylated CHO cells and a force-elongation curve obtained. The required force for pulling the tether (protrusion force) was found to be around 50 pN and maximal tether lengths of around 20 microns.
Aresis tweezers were employed to induce high curvature in membrane protrusions and establish the spatial distribution of specific ion channels (Piezo1) in the plasma membrane of living cells.
Selected related references:
Membrane curvature governs the distribution of Piezo1 in live cells; Shilong Yang et al 2022 Nat Comm 13, 7467; DOI: 10.1038/s41467-022-35034-6 LINK
Structural Rearrangements in CHO Cells After Disruption of Individual Cytoskeletal Elements and Plasma Membrane; Špela Zemljič Jokhadar and Jure Derganc 2015 Cell Biochem Biophys 71, 1605–1613; DOI: 10.1007/s12013-014-0383-9 LINK
Cytoskeleton Modification and Cholesterol Depletion Affect Membrane Properties and Caveolae Positioning of CHO Cells; Maja Grundner et al 2014 J Membrane Biol 247, 201–210; DOI: 10.1007/s00232-013-9625-9 LINK
Other publications related to cell mechanics experiments performed with Aresis solutions:
Targeted perforation of vesicles by optical tweezers: Locally induced shockwaves for selective perforation of cargo loaded lipid vesicles with temporal and spatial control; Jure Derganc et al 2023 RSC Adv., 13, 24830–24834; DOI: 10.1039/d3ra03988a LINK
Force Mapping during the Formation and Maturation of Cell Adhesion Sites with Multiple Optical Tweezers; Melanie Schwingel and Martin Bastmeyer 2013 PLoS One., 8: e54850; DOI: 10.1371/journal.pone.0054850 LINK